Industry
HIGHLIGHTS
- AI, GenAI, and agentic AI bring in fundamental shift in how pharma and medtech conduct research, operations, and regulatory engagements, creating innovation opportunities and improving patient outcomes.
- This shift is driven by the need for greater autonomy and proactive problem-solving capabilities within complex workflows where agentic AI is better than AI or GenAI.
- The path to gaining a competitive edge in this evolving landscape will depend on how organizations and their leaders proactively harness agentic AI to improve efficiency, compliance, streamline costs, and improve patient outcomes.
On this page
Agentic AI advantage
Agentic artificial intelligence (AI) is redefining the pharma and med tech value chain.
AI and generative AI (GenAI) have created unprecedented opportunities for efficiency and innovation in the pharma and med tech industries, transforming every aspect—right from research and development (R&D) to patient engagement. GenAI accelerates the identification of novel drug targets, designs optimized molecules, simplifies complex clinical trial processes, and even drafts personalized patient communications, all while continuously learning and adapting. This big shift promises to shorten the decades-long development cycles to years and months, enhance the precision of personalized medicines, and fundamentally reshape the industry by accelerating the delivery of life-saving therapies to patients.
The pharmaceutical and medtech industries are progressively moving beyond the initial applications of AI and GenAI, which primarily focused on data analysis, pattern recognition, and content generation, towards the more transformative realm of agentic AI. This shift is driven by the need for greater autonomy and proactive problem-solving within complex workflows. While GenAI excels at creating new data, molecules, or content based on prompts, agentic AI goes a step further by developing systems that can interpret situations, reason through scenarios, set sub-goals, and execute multi-step actions independently. Agentic systems are designed to learn from new data all the time, turning every experiment into a valuable source of incremental knowledge. AI agents operate based on data and algorithms, minimizing potential human biases in the selection and optimization process.
The value chain play
Agentic AI does not merely generate insights or content, it can also autonomously plan, execute, and adapt workflows for the entire value chain in pharma and medtech. Agentic AI can enable autonomous workflows in drug discovery and development, regulatory, manufacturing, supply chain, and marketing functions, enhancing efficiency and patient outcomes.
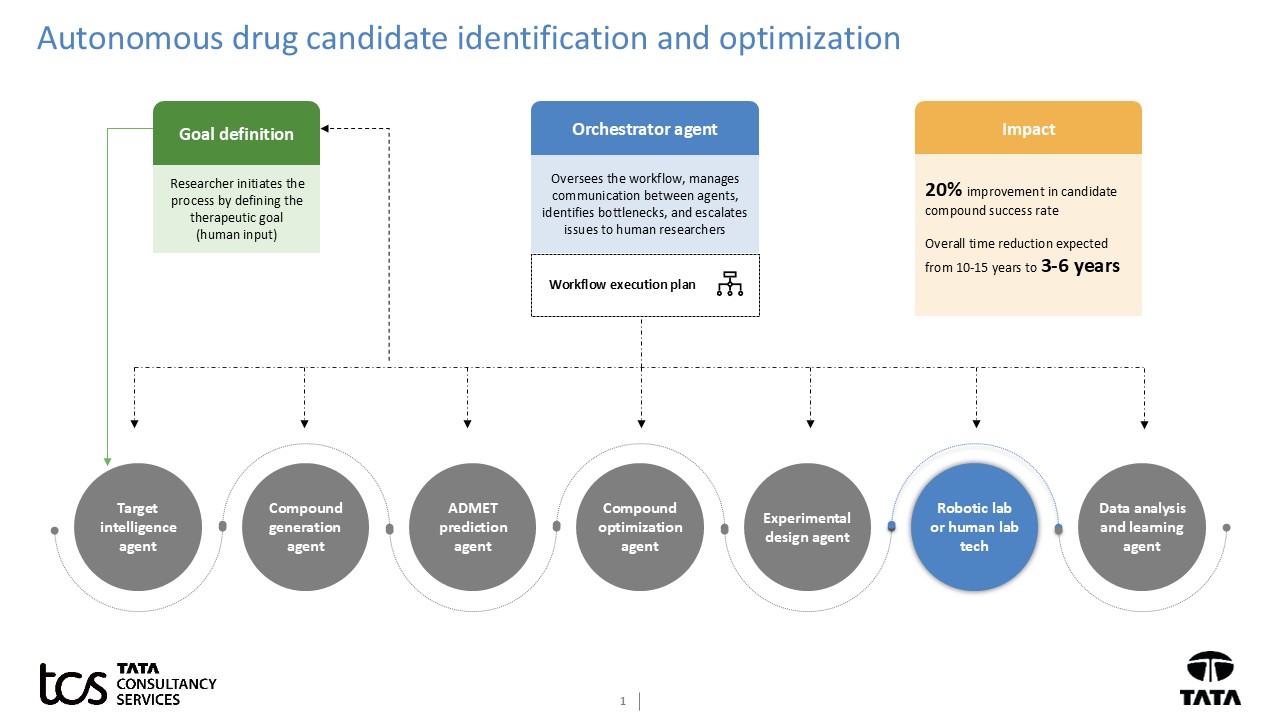
Let’s look at one example of how an agentic AI workflow for autonomous drug candidate identification and optimization will get orchestrated. The workflow will involve several specialized AI agents collaborating under an orchestrator agent, forming a continuous feedback loop.
Generative agents can generate hundreds of thousands of new chemical entities, which is beyond the capability of humans. The agents can explore the vast chemical spaces to identify safe and effective drug-like molecules. The autonomous nature of, and rapid communication between, agents will drastically reduce the time needed for each design test learn cycle. Fewer expensive and time-consuming wet-lab experiments are needed due to improvement in in-silico prediction and optimization, leading to cost savings. Continuous learning and adaptation based on experimental feedback can lead to more informed decisions and a higher likelihood of identifying viable drug candidates.
This agentic AI workflow represents a significant shift from traditional, sequential drug discovery processes to a more dynamic, intelligent, and autonomous approach. This holds immense promise for bringing life-saving medicines to patients faster and more efficiently.
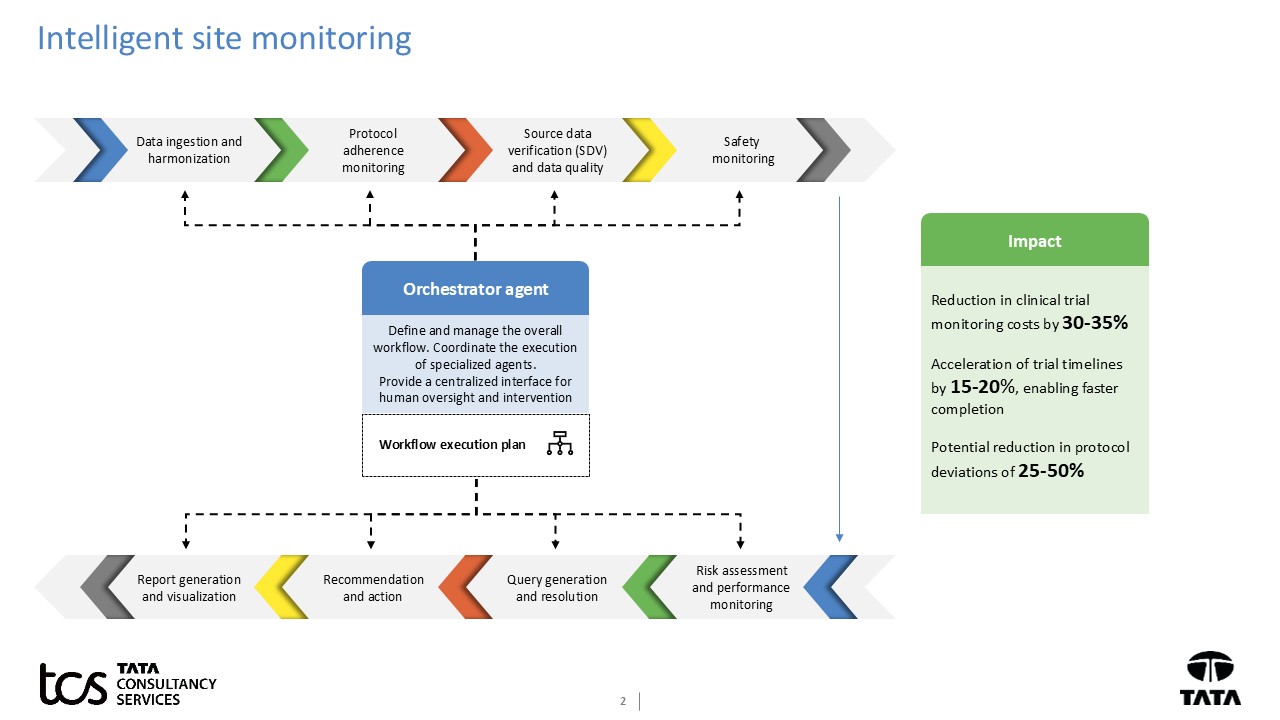
Similarly, clinical trial monitoring is a key activity, which ensures timely and successful completion of clinical trials with good quality data.
Agentic AI-based intelligent site monitoring fundamentally shifts clinical trial oversight from a reactive, manual, and often inefficient process to a proactive, automated, and intelligent site monitoring.
This will enable clinical trial sites to ensure data quality, adhere to protocols, ensure patient safety, and operational efficiency. This reduces the need for extensive manual oversight and speeds up trial timelines and, ultimately, ensures quicker access to new life-saving medicines.
New business models
The agentic AI transformation is poised to trigger novel business models in pharma and medtech, shifting focus from a product-centric approach to value-based, patient-centric solutions. New business models for AI-powered personalized medicine, diagnostic tools, and drug repurposing platforms will emerge with the rise of digital therapeutics (DTx), software as a medical device (SaMD), and remote patient monitoring platforms.
One significant emerging model is ‘healthcare as a service’, where companies will offer continuous, personalized monitoring and intervention platforms rather than just selling drugs or devices. Agentic AI, acting as virtual case managers or digital health companions, will enable proactive patient engagement, adherence management, and real-time health interventions, creating subscription-based revenue streams. Another model involves ‘precision therapeutics as a service’, where the focus moves beyond a single drug to an integrated offering that combines an AI-designed personalized therapy with continuous monitoring and adaptive dosing adjustments powered by agentic systems.
Furthermore, agentic AI will facilitate ‘data monetization and insights as a service’, where companies can leverage the vast amount of real-world data collected by their agents to provide valuable insights to payers, providers, and researchers, creating new revenue streams from high-fidelity, actionable intelligence. This transition emphasizes moving towards outcome-based contracts and integrated solutions that leverage the autonomous capabilities of AI agents to deliver continuous value throughout the patient journey.
The future landscape
Multiple considerations mark the way forward for the technology and outsourcing strategy. A strong technology foundation and FAIR (findable, accessible, interoperable, reusable), data ecosystem is the base that will enable a long-term successful agentic AI deployment. Standardization of metadata, application programming interfaces (APIs), and data ontologies enabling interoperability are key to an AI and agentic-ready data ecosystem. Organizations that fail to integrate enterprise data platforms or use inconsistent data definitions will face perpetual bottlenecks. Organizations must invest in enterprise architecture that supports modular, plug-and-play data and AI capabilities.
AI is driving a big shift towards using cloud computing, big data analytics, and advanced machine learning systems. Strategic partnerships with AI technology vendors, cloud hyperscalers, and startups are helping pharma and medtech firms access state-of-the-art tools without building everything in-house.
Traditional contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) may need to incorporate agentic-powered solutions to remain competitive. The emergence of AI agents that can automate tasks and work together across different processes will change and transform operations and outsourcing models. Outcome-based, AI-augmented service delivery models can replace traditional full-time equivalent (FTE)-based business outsourcing. There will be emergence of augmented experts powered by large language models (LLMs) and intelligent assistants to execute knowledge-based tasks like clinical statistical programming, medical writing, safety case processing and others.
Navigating the challenges and mitigating risks
Bringing agentic AI into pharma and medtech comes with its own set of challenges and risks, like keeping data private and secure, avoiding biases in algorithms, and protecting against cybersecurity threats. Global drug regulators, such as the Food and Drug Administration (FDA), USA, and European Medicines Agency (EMA), European Union, are either working on, or have already released, guidelines on how AI can be used in life sciences. The evolving regulatory landscape also requires companies to adapt to new guidelines. Developing transparent and explainable AI algorithms is crucial for responsible use in life sciences. Copyright and intellectual property infringement are also significant risks to consider.
GenAI-generated documents, especially those used for regulatory submissions or clinical content, must undergo rigorous validation and human-in-the-loop review to ensure scientific integrity and compliance. AI systems manage large volumes of patient data, making them targets for cyber threats. Organizations must ensure robust data governance and encryption practices while ensuring compliance with global data protection laws such as General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA).
AI, GenAI models that are trained on biased datasets can produce biased results. It is crucial to have cross-functional AI ethics and oversight committees to evaluate if AI deployments are ethical and accountable.
Strategic road map
Early and strategic adoption of agentic AI can provide a significant competitive advantage in pharma and medtech. There is a need to revisit organizational operating models and cultures investing in technology and adapting talent strategies. Building strong internal AI capabilities through talent acquisition and upskilling is also crucial for strategic advantage.
To lead in the future, pharma and medtech organizations must take a structured approach to enable an organization-wide adoption and implementation of agentic AI, aligning it with business goals and ensuring effective use of AI technologies. The road map should address the ‘what’, ‘why’, and ‘how’ of AI implementation. A comprehensive AI strategy includes defining an AI vision, building digital foundations, prioritizing use cases, ensuring ethical AI, fostering innovation culture, strengthening external ecosystems, and upskilling the workforce.
Agentic AI is impacting the pharma and medtech industries, representing a fundamental shift in operations across their value chain. Potential benefits for organization are substantial: from accelerating drug discovery, product designs, and optimizing clinical trials to transforming manufacturing and supply chains and ensuring regulatory compliance.
This revolution promises to address long-standing bottlenecks, improve productivity, and change how operations are run creating new avenues for growth and innovation. The need to adopt these technologies is driven by the growing pressure to lower development costs, speed up the time to market, and provide more patient-focused effective therapies.
Organizations must proactively address the challenges and risks associated with adoption of agentic AI. Getting involved with AI and GenAI early and strategically is key to staying ahead and maintaining a lasting competitive edge. An integrated ecosystem approach encompassing technology, organizational change, talent development, and strategic partnerships will enable pharma and medtech companies to navigate the intelligent revolution and achieve future success.